1. INTRODUCTION
1.1 GENERAL
Concrete is the world’s most versatile,
durable and reliable construction material. Large quantities of Portland cement
are required for concrete. The consumption of Ordinary Portland Cement (OPC)
causes pollution to the environment due to the emission of CO2. Geopolymer concrete was introduced to reduce environmental
pollution that causes by production of Portland cement.
In 1978, Professor Joseph Davidovits
introduced the development of mineral binders with an amorphous structure,
named geopolymers. Davidovits (1988; 1994) proposed that an alkaline liquid
could be used to react with the silicon (Si) and the aluminium (Al) in a source
material of geological origin or in by-product materials such as fly ash and
rice husk ash to produce binders. Because the chemical reaction that takes
place in this case is a polymerization process, he coined the term ‘Geopolymer’
to represent these binders. This was a class of solid materials, produced by
the reaction of an alumino silicate powder and an alkaline liquid. The initial
goal for the research done on these geopolymers was to find a more fire
resistant binder material due to the high amount of fires in Europe at that
time. This research led to the material being used as coatings for the fire
protection of cruise ships and thermal protect results in a low flexural
strength. Brittleness of both concrete types is compensated by conventional
steel reinforcement.
Geopolymer concrete is an innovative
construction material which shall be produced by the chemical action of
inorganic molecules. Otherwise geopolymer is an inorganic alumino- hydroxide
polymer synthesized from predominantly silicon (Si) and aluminium (Al)
materials of geological origin or byproduct materials such as fly ash. The term
Geopolymer was introduced to represent the mineral polymers resulting from
geochemistry. The process involves a chemical reaction under highly alkaline
conditions on Si-Al minerals, yielding polymeric Si-O-Al-O bonds in amorphous
form. Due to its high mechanical properties combined with substantial chemical
resistance (magnesium or sulphate attack), low shrinkage and creep and
environment friendly nature (very less amount of CO2 production in comparison
with OPC), it is a better construction material for future.
1.2 NECESSITIES OF GEOPOLYMER CONCRETE
This type of geopolymer concrete is starting
to revolutionize concrete. It is being used more in highway construction
projects and offshore applications. Construction is one of the world wide
growing fields. As per the present world statics, every year million tons of
cement are required. Ordinary Portland cement is commonly used in concrete.
While producing one ton of cement, approximately one ton of carbon di oxide
will be emitted to the atmosphere, which cause major problems in environment.
Also huge quantity of energy is also required for the production of cement.
Hence it is most essential to find an alternative binder. The Thermal Industry
produces a waste called fly ash which is simply dumped on the earth, occupies
large areas. The waste water from the Chemical Industries is discharged into
the ground which contaminates ground water. By producing Geopolymer Concrete
all the above mentioned issues shall be solved by rearranging them.
Waste Fly Ash from Thermal Industry + Waste
water from Chemical Refineries = Geo polymer concrete.
Since Geopolymer concrete doesn’t use any
cement, the production of cement shall be reduced and hence the pollution of
atmosphere by the emission of carbon di oxide shall also be minimized.
2. GEOPOLYMER CONCRETE
2.1 GEOPOLYMER THEORY
Geopolymerization is a geosynthesis, a
reaction that chemically integrates minerals. The exposure of aluminosilicate
materials such as fly ash, blast furnace slag, or thermally activated substances
to high-alkaline environments (hydroxides, silicates) gives rise to the
formation of a geopolymer. Geopolymers are characterized by a two- to
three-dimensional Si-O-Al structure.
Dissolution occurs immediately upon contact
between the alkaline solution and the pozzolanic material and allows for ionic
interface between species and the breaking of covalent bonds between silicon,
aluminium and oxygen atoms. The rate of dissolution is relevant to the amount
and composition of the ashes and the pH of the activating solution.
The polymerization process involves a substantially fast chemical reaction under alkaline conditions on Si-Al minerals, resulting in a three-dimensional polymeric chain and ring structure consisting of Si-O-Al-O bonds. The formed gel product contains alkaline cations which compensate for the deficit charges associated with the aluminium for silicon substitution. An intermediate, aluminium rich phase is first formed which then gives way to a more stable, silicon- rich three-dimensional gel product, which is dependent upon curing conditions and activator type.
2.2 GEOPOLYMER DEVELOPMENT
Geopolymer cements develop through a series
of several distinct reaction processes from initial pozzolanic activation to
final microstructure development. The major processes involved are dissolution
of the alumino silicate species within a highly basic, alkaline environment,
polymerization of the dissolved minerals into short-lived structural gel,
precipitation of formed hydration products similar to natural zeolites and
final hardening of the matrix by excess water exclusion and the growth of
crystalline structures. Figure 2.1 shows the overall polymerisation process in
alkali activated geopolymer concrete.
Fig.2.1
Geopolymer development
2.3 CONSTITUENTS OF GEOPOLYMER CONCRETE
2.3.1 Coarse Aggregate
Coarse aggregates used in case of cement
concrete can be used in case of Geopolymer concrete (GPC) also where the coarse
aggregate should conform to IS-383- 1970.
2.3.2 Fine Aggregate
In place of sand we can also use bottom ash
which can be a replacement of sand. Up to a level of 20% replacement of sand
gives a good compressive strength.
2.3.3 Admixtures
To improve the workability of fresh concrete,
a commercially available naphthaline based superplasticizer was used. Lignin
based first generation plasticizer shows better performance in terms of
workability over third generation superplasticizer. High range water reducing
naphthalene based super plasticiser, naphthalene sulphonate based superplasticizer
etc was also use.
2.3.3 Alkaline Activators
According to Prof. J. Davidovits the alkaline
liquid should be made prior to one day before mixing because at the time of
mixing of Na2SiO3 with NaOH
solution it generates a huge amount of heat and the polymerization takes place
by reacting with one another, which will act as a binder in the geopolymer
concrete. Common activators include NaOH, Na2SO4 , waterglass, Na2 CO3 , K2 CO3 , KOH, K2SO4 and cement clinker, the most utilized alkaline activators are a
mixture of sodium or potassium hydroxides (NaOH, KOH) and sodium waterglass
(nSiO2 Na2 O) or potassium
waterglass (nSiO2 K2 O).
Sodium Hydroxide (NaOH)
NaOH is also commonly used as an alkaline
activator in geopolymer production. Generally NaOH is available in market in
the form of pellets or flakes form with 96% to 98% purity where the cost of the
product depends on the purity of the material. The solution of NaOH was formed
by dissolving it in water with different molarity. It is recommended that the
NaOH solution should be made 24 hours before casting and should be used with 36
hours of mixing the pellets with water as after that it is converted to
semi-solid state.
The concentration and molarity of this
activating solution determines the resulting paste properties. While high NaOH
additions accelerate chemical dissolution, it depresses ettringite and CH
(carbon-hydrogen) formation during binder formation. Furthermore, higher
concentrations of NaOH promote higher strengths at early stages of reaction,
but the strength of aged materials were compromised due to excessive OH in
solution causing undesirable morphology and non-uniformity of the final
products. It is found that geopolymers activated with sodium hydroxide develop
greater crystallinity thus improving stability in aggressive environments of
sulphates and acids.
Potassium Hydroxide (KOH)
KOH has been found to produce high
compressive strengths and improved porosity in geopolymer cements. Since K+ is
more basic than other activating ions, it possesses a greater potential for
polymeric ionization in solution resulting in high reactivity of the prime
pozzolan, a denser final product and a matrix formation capable of achieving
increased compressive strength values. NaOH actually possesses a greater
capacity to liberate silicate and aluminate monomer.
Sodium Silicate (Na2SO3) and potassium silicate
Sodium silicate is also known as waterglass
which is available in the market in gel form. The ratio of SiO2 and Na2O in sodium silicate gel highly affects the
strength of geopolymer concrete. Mainly it is seen that a ratio ranging from 2
to 2.5 gives a satisfactory result.
Sodium or potassium silicates are
manufactured by using sand (SiO2) with sodium or potassium carbonate (Na2 CO3 or K2 CO3 ) at temperatures in excess of 1100 °C and dissolving the product
with high pressure steam into a semi-viscous liquid referred to as waterglass.
Waterglass is rarely used as an independent activating unit, because it does
not possess enough activation potential to initiate pozzolanic reaction alone.
It is commonly mixed with NaOH or KOH as a strengthening agent to enhance
alkalinity and increase overall specimen strength. The most common alkaline
liquid used in geopolymerization is a combination of sodium hydroxide or
potassium hydroxide and sodium silicate or potassium silicate.
2.3.6 Geopolymer
Geopolymers are formed by alkali-activating a
variety of materials including fly ash, blast furnace slag, thermally activated
clays etc. to produce a cement-like material. The three most common raw binders
used in geopolymerisation are slag, calcined clays (metakaolin) and coal fly
ash. The binder materials should contain high levels of aluminium (Al) and
silicon (Si) in amorphous form. The raw materials play a significant role in
the geopolymer reaction and affect the mechanical properties and microstructure
of the final geopolymeric products.
Generally, materials containing mostly
amorphous silica (SiO2) and alumina (Al2O3) are the source for geopolymer production.
Naturally available materials like kaolin , natural puzzolana and Malaysian
marine clay , treated minerals like metakaoline and waste materials like fly
ash ,Construction waste , red clay brick waste , fly ash and rice husk-bark
ash, fly ash and blast furnace slag etc can be used. Many different materials
have already been investigated and used as the binder in geopolymer concrete
mixes, including:
Ø Low calcium fly ash ( Class F fly-ash)
Ø High calcium fly ash (Class C fly-ash)
Ø Calcined kaolin or metakaolin
Ø Natural minerals containing Al and Si
Ø Silica fume
Ø Slag
Ø Red mud
Ø Albite
Metakaolin was widely used as the binder in
the early stages, but due to its flat shape it tends to have high water demand.
Fly ash particles have a rounder shape, ensuring more promising workability and
a low water demand.
3. GEOPOLYMER CATEGORIES
There are currently four different geopolymer
categories including:
Ø Slag based geopolymer
Ø Rock based geopolymer
Ø Fly ash based geopolymer
Ø Ferro-sialate based geopolymer
3.1 SLAG BASED GEOPOLYMER
The first geopolymer developed was a slag
based geopolymer in the 1980s. The reason for using this type of cement is due
to its the rapid strength gain as it can reach strengths of up to 20 MPa after
just 4 hours. Slag is a partially transparent material and a by-product in the
process of melting iron ore. It usually consists of a mixture of metal oxides
and silicon dioxide. It is also used in the cement and concrete industry. The
substitution of OPC with slag is one of the many benefits that it provides to
OPC concrete, reducing life cycle costs and improving the workability of the
fresh concrete, Easier finishability, higher compressive and flexural strength
and also the improved resistance to acid materials. The reactions of slag in
alkali activating systems and in cement blends are dominated by the small
particles. The particles that are above 20 µm usually react slowly, while
particles under 2 µm react completely within 24 hours. Thus, when slag is used
in geopolymerisation, careful control of the particle size distribution must be
ensured to control the strength of the binder.
Examples of slag used are; Iron blast-furnace
slag, Corex slag& Steel slag.
3.2 ROCK BASED GEOPOLYMER
To compose this type of geopolymer, a
fraction of the MK-750(“MK” is an abbreviation for metakaolin and the “750”
represents the temperature at which it was produced) in the slag based
geopolymer is replaced by natural rock forming materials such as feldspar and
quarts. This mixture yields a geopolymer with better properties and less CO2 emissions than that of the ordinary slag based geopolymer. The
components of rock based geopolymer cement is metakaolin MK-750, blast-furnace
slag, natural rock forming materials (calcined or non-calcined) and a user
friendly alkali silicate.
3.3 FLY ASH BASED GEOPOLYMER Fly ash is the waste material produced in blast furnace. Components of fly ash are amorphous composition (60%), quartz (20%), mullite (17%), maghemite (1.7%) and hematite (.9%).
3.3 FLY ASH BASED GEOPOLYMER Fly ash is the waste material produced in blast furnace. Components of fly ash are amorphous composition (60%), quartz (20%), mullite (17%), maghemite (1.7%) and hematite (.9%).
Fly ash is commonly used as a substitute for
OPC in concrete and the addition of it provides;
Ø Fly ash consists of spherical particles which improve the workability of the fresh OPC concrete. This enables one to reduce the amount of water in the mix which reduces the amount of bleeding of OPC concrete.
Ø Improves mechanical properties such as compressive strength, due to the water reduction and ensures a higher reactiveness and better “packing” of particles.
Ø Reduce the cost of the OPC concrete.
Ø Reduces the CO2 emissions and drying shrinkage.
Ø Smoother surface. .
Fig. 2.2
Graded fly ash
Fly ash can be divided in to two, type F fly
ash and type C fly ash. The type F fly ash can be again classified into two
Alkali-activated fly ash geopolymer and Fly ash/slag based geopolymer. most of
the globally available fly ash material is a low calcium by product obtained
from the burning of anthracite and bituminous coal.
Alkali-activated fly ash geopolymer
This kind of geopolymer usually requires heat
curing at 60 ºC to 80 ºC. It is also known as the alkali activation method. A
high concentration of sodium hydroxide solution is required to ensure an
adequate geopolymerisation process. The mixture consists of fly ash and a
user-hostile sodium hydroxide solution. The fly ash particles are embedded into
an aluminosilicate gel with a Si: Al ratio of 1 to 2.
Fly ash/slag based geopolymer
This kind of geopolymer is more user-friendly
and it hardens at room temperature. The mixture consists of a user-friendly
silicate, blast furnace slag and fly ash. The fly ash particles are embedded
into a geopolymer matrix with and Si: Al ratio of 2.
3.4 FERRO SIALATE BASED GEOPOLYMER
This type of geopolymer has the same
properties as rock based geopolymers but contains geological elements with high
iron oxide content, giving the geopolymer a red colour. Some of the aluminium
atoms in the matrix are substituted with iron ions to yield a poly
(Ferro-sialate) type geopolymer with the following formation: (Ca,K)-
(-Fe-O)-(-Si-O-Al-O-).
4. STUDY OF PROPERTIES OF GEOPOLYMER CONCRETE
4.1 STUDY OF FLY ASH BASED GEOPOLYMER CONCRETE
4.1.1Creep Test
Test Specimens
Test specimens for the creep test were
150x300 mm cylinders and eight cylinders were prepared for each test. Three
cylinders were used for measuring the creep, two companion cylinders measured
the drying shrinkage and the other three cylinders were used for the
compressive strength test.
Test Procedure
The three specimens for creep test were placed
in a specially-built creep testing frame with a hydraulic loading system.
Before the creep specimens were loaded, the 7th day compressive strength of
geopolymer concrete was determined by testing the three cylinders reserved for
the compressive strength test. The creep specimens were applied with a load
corresponding to 40 percent of the measured mean compressive strength of
concrete. This load was maintained as the sustained load throughout the
duration of the test. The strain values were measured and recorded immediately
before and after the loading. Strains experienced by the control shrinkage
specimens were measured at the same time as the strain measurements on creep
specimens. The strain values were measured and recorded at 2 hours, 6 hours,
and then every day for the first week, after loading. The measurements then
continued once a week until the fourth week. After that, the measurements were
done once in 2 weeks until the twelfth week and the once every four weeks until
one year. The creep tests were conducted in a laboratory room where the
temperature was maintained at about 23ºC, but the relative humidity could not
be controlled. The relative humidity varied between 40% and 60% during the
test.
Fig.4.1
creep test
In OPC concrete creep is higher than that of
geopolymer concrete and thus the geopolymer concrete specimens undergo low
creep.
4.1.2 Drying Shrinkage Test
Test specimen
75x75x285 mm prisms with gauge studs were
used for drying shrinkage test.
Test Procedure
The shrinkage strain measurements started on
the third day after casting the concrete. On the third day after casting, the
specimens were demoulded and the first measurement was taken. Horizontal length
comparator was used for length measurements. The next measurement was on the
fourth day of casting, considered as Day 1 for the drying shrinkage
measurements. The measurements then continued every day in the first week, once
a week until the fourth week, once in two weeks until the twelfth week, and then
once in four weeks until one year. During the drying shrinkage tests, the
specimens were kept in a laboratory room where the temperature was maintained
at approximately at 23ºC. The relative humidity of the room varied between 40%
and 60%.
Fig. 4.2
Horizontal Length Comparator with Drying Shrinkage Test Specimen
Geopolymer concrete specimens undergo low
drying shrinkage. The drying shrinkage of geopolymer concrete, cured at ambient
temperatures, shows shrinkage significantly higher than that of heat cured
geopolymer concrete. The excess water in the geopolymer concrete evaporates
during the heat curing process, eliminating almost any chance of drying
shrinkage. The drying shrinkage for geopolymer concrete cured at ambient
temperatures is similar compared to that of OPC concrete. Figure 4.3 shows the
drying shrinkage of heat cured and ambient curing specimens.
Fig.4.3
Drying shrinkage of heat cured and ambient cured specimens
4.1.3 Sulphate Resistance Test
Test Procedure
The test specimens were immersed in sulphate
solution on the 7th day after casting. Sodium sulphate (Na2 SO4) solution with 5% concentration was used as
the standard exposure solution for all tests. The specimens were immersed in
the sulphate solution in a container, the volume proportion of sulphate
solution to specimens was four to one. In order to maintain the concentration,
the solution was replaced every month.
Fig.4.4
Specimens Soaked in Sodium Sulphate Solution
4.1.4 Acid Resistance Test
Sulphuric acid is one type of acid solution
that is frequently used to simulate the acid attack in sewer pipe systems. In
such systems, sulphuric acid attack is a particular problem as it is generated
bacterially from hydrogen sulphide. To test the acid resistance of geopolymer
concrete, the specimens be exposed to sulphuric acid solution with a
concentration of pH = 1. To evaluate the acid resistance of fly ashbased
geopolymer concrete, the specimens were soaked in sulphuric acid solution with
selected concentrations ranging from 0.25% to 2% with the measured pH ranges
from about 0.9 to 2.1, up to one year of exposure. The test specimens were
immersed in sulphuric acid solution in a container; the ratio of the volume of
the acid solution to the volume of the specimens was 4. The solution was
stirred every week and replaced every month.
4.1.5 Compressive Strength
It has been confirmed that geopolymer
concrete can reach significantly high compressive strengths when cured either
by heat activation or at ambient temperatures. Heat activation is necessary to
accelerate the geopolymerisation process and therefore higher compressive
strengths will be achieved when heat activated, compared to ambient curing. The
addition of slag in the matrix improves the compressive strength of the
geopolymer concrete significantly when cured at ambient temperatures.
The test results plotted in Figure 4.6 show
that the 7th day compressive strength of ambient-cured geopolymer concrete and
the subsequent strength gain with respect to age depend on the ambient
temperature at the time of casting. The 7th day compressive strength of fly
ash-based geopolymer concrete increased as the average ambient temperature at
casting increased. Also, the compressive strength of ambientcured geopolymer
concrete significantly increased with the age.
Fig. 4.5
Compressive Strength of Geopolymer Concrete Cured in Ambient Condition
4.1.6 Modulus Of Elasticity And Poisson’s Ratio
Tests have been conducted on the
stress-strain relationship of low calcium fly ash based geopolymer concrete and
it showed similar results to that of OPC concrete. The stress-strain curve of
geopolymer concrete agrees well with the predictions that were originally
developed for OPC concrete. As for OPC concrete, the modulus of elasticity
increased with the increase of compressive strength. According to previous
research it is suggested that fly ash based geopolymer concrete, cured at
elevated temperatures, yields higher modulus of elasticity values compared to
fly ash/slag based geopolymer concrete that cures at ambient temperatures.The
Poisson’s ratio of the geopolymer concrete specimens, with compressive
strengths ranging between 40 MPa and 90 MPa, was similar to that of OPC
concrete. But the modulus of elasticity can be brought equal to that of OPC
concrete by selecting the appropriate aggregate content as well as the optimum
fine aggregate to total aggregate ratio.
Table 4.1
Young’s Modulus and Poisson’s Ratio
Static modulus of elasticity for various mixes is given as;
Table 4.2
static modulus of elasticity for various mixes.
Where; Ec = ∆σ/∆
∆σ and ∆ are the increase in stress and strain respectively.
∆σ and ∆ are the increase in stress and strain respectively.
For OPC, EC =5000√fck N\mm2.
For GPC, EC =4600√fck N\mm2.
The stress-strain curve of OPC&GPC for various mixes are given
by,
Fig.4.6
Stress-Strain curves for OPC & GPC
4.2 STUDY ON PROPERTIES OF GEOPOLYMER CONCRETE USING
GLASS FIBER.
4.2.1 Experimental Investigation
The experiment is conducted to find out the
parameters that influences the mixture proportions and the properties of low
calcium fly ash- based geo polymers concrete and as in the case of OPC the
aggregates occupied 75- 80 % of the total mass of concrete. In order to
minimize the effect of the properties of the aggregates on the properties of
fly ash based geo polymers concrete.
4.2.2 Preparations Of geopolymer Concrete
Sodium Hydroxide solution
Sodium Hydroxide pellets are taken and
dissolved in water at the rate of 16 molar concentrations. It is strongly
recommended that the sodium hydroxide solution must be prepared 24 hours prior
to use and also if it exceeds 36 hours it terminate to semi solid liquid state.
So the prepared solution should be used within this time.
Molarity Calculation
The solids must be dissolved in water to make
a solution with the required concentration. The Concentration of sodium
hydroxide solution can vary in different molar. The mass of NaOH solids in a
solution varies depending on the concentration of the solution. For instance,
NaOH solution with a concentration of 16 molar consists of 16 x 40 = 640grams
of NaOH solids per liter of water, were 40 is the molecular weight of NaOH.
Note that the mass of water is the major component on both the alkaline
solutions. The mass of NaOH solids was measured as 444 grams per Kg of NaOH
solution with Concentration of 16 molar.
Fiber Proportion
Glass fibre of 0.5%, 1%, 1.5% and 2% is used
here to find out the optimum dosage of glass fibre based on its compressive,
tensile and flexural test results. For M 25 grade conventional concrete and
geopolymer concrete the compressive strength, tensile strength and load
deflection test results are conducted. GPC values are higher than conventional
concrete higher concentration (in terms of molar) of sodium hydroxide solution
results in higher compressive strength of fly ash-based geopolymer concrete.
4.3 Compressive Strength
Compressive strength is one of the important
properties of concrete. Concrete cubes of size 150mmx150mmx150mm were cast with
Glass fibers. After 24 hours, the specimens were demoulded and subjected to
water curing. After 28 days of curing specimens were taken and allowed to dry
and tested in compressive strength testing machine.
Table 4.3 compressive strength (% fiber
added)
Fig.4.7 Compressive strength chart
Split tensile strength is indirect way of
finding the tensile strength of concrete by subjecting the cylinder to a
lateral compressive force. Cylinders of size 150mm diameter and 300mm long were
cast with Glass fibers. After 24 hours the specimen were demoulded and
subjected to water curing. After 28 days of curing of specimens were taken and
allowed to dry and tested in universal testing machine by placing the specimen
horizontal.
Split tensile strength, fsp = 2P/π bd
Where, P = Load applied to the specimen in N
b =
Breadth of the specimen in mm
d = Depth of the specimen in mm
Table 4.4
split tensile strength (% fiber added)
Fig.4.8
Split tensile strength chart
4.5 Flexural Strength
Ø Flexural strength of a concrete is a measure of its ability to resist bending. Flexural strength can be expressed in terms of ‘modulus of rupture’.
Ø Concrete specimens for flexural strength were cross sectional area of 150mm width with 150mm depth and length of 700mm concrete beam.
The specimen is subjected to bending, using
four point loading until it fails. The distance of the loading point is 150mm
and the supporting point (L) is 450mm.
The flexural strength of concrete = P x L
x 1000 / B x D
Where,
P - Maximum load applied to the specimen in
KN.
L - Length of the specimen in mm. B - Width
of the specimen in mm
D - Diameter of the specimen in mm.
Table 4.5
Flexural strength (% fiber added)
Fig.4.9
Flexural strength chart
Result Obtained
Ø Compressive strength of 1% glass fiber reinforced concrete has found to be 10% increase in strength, when compared to that of Conventional concrete.
Ø Split tensile strength of 1% glass fiber reinforced concrete has found to be 10% increase in strength, when compared to that of Conventional concrete.
Ø Flexural strength of 1% glass fiber reinforced concrete has found to be 20% increase in strength, when compared to that of Conventional concrete.
5. ADVANTAGES OF GEOPOLYMER CONCRETE
5.1 High Strength
It has a high compressive strength that
showed higher compressive strength than that of ordinary concrete.it also has
rapid strength gain and cures very quickly. Making it an excellent for quick
builds. Geopolymer concrete has high tensile strength.it is less brittle than
Portland concrete and can withstand more movement. it is not completely earth
quake proof, but does withstand the earth moving better than traditional
concrete.
5.2 Very Low Creep And Shrinkage
Shrinkage can cause severe and even dangerous
cracks in the concrete from the drying and heating of the concrete or even the
evaporation of water from the concrete. Geopolymer concrete does not hydrate;
it is not as permeable and will not experience significant shrinkage. The creep
of geopolymer concrete is very low. The tendency of the concrete become
permanently deformed due to the constant forces being applied against it is
known as creep in concrete.
5.3 Resistant To Heat And Cold
It has the ability to stay stable even at
temperature of more than 2200 degrees Fahrenheit. Excessive heat can reduce the
stability of concrete causing it to spall or have layers break off. Geopolymer
concrete does not experience spalling unless it reaches over 2200 degrees
Fahrenheit.As for old temperatures, it is resistant to freezing. The pores are
very small but water can still enter cured concrete. When temperature dip to
below freezing that water freezes and then expands this will cause cracks to
form. Geopolymer concrete will not freeze.
5.4 Chemical Resistance
It has a very strong chemical resistance.
Acids, toxic waste and salt water will not have an effect on geopolymer
concrete. Corrosion is not likely to occur with this concrete as it is with
traditional Portland concrete.
5.5 Environmental Benefits
The use of geopolymer concrete reduces the need of OPC, that’s why we have to reduce the environmental pollution that caused by the emission of CO2 during the production of OPC.
6. Geopolymer Concrete shall be used in repairs and rehabilitation works.
6. DISADVANTAGE OF GEOPOLYMER CONCRETE
6.1 Difficult To Create
Geopolymer concrete requires special handling
needs and is extremely difficult to create. It requires the use of chemicals,
such as sodium hydroxide, that can be harmful to humans.
6.2 Pre Mix Only
Geopolymer concrete is sold only as a
pre-cast or pre mix material due to the dangers associated with creating it.
6.3 Geopolymerization Process Is Sensitive
This field of study has been proven
inconclusive and extremely volatile. Uniformity is lacking.
7. APPLICATIONS OF GEOPOLYMER CONCRETE
Geopolymer mortars and concretes possess a
high potential for use in commercial applications due to their enhanced
durability, thermal and chemical
22 resistance properties, rapid development of mechanical strength, adherence to reinforcements aggregates and economic benefit as an industrial by-product material.
22 resistance properties, rapid development of mechanical strength, adherence to reinforcements aggregates and economic benefit as an industrial by-product material.
7.1 Concrete Pipes The use of geopolymer concretes for commercial sewer piping is a good option from the basis of their resistance to sulphates and acidic products. Sulphuric acid is generated in conventional sewer systems through the breakdown of hydrogen sulphide by aerobic bacteria in the system and is the main factor in corrosion and structural deterioration of the piping networks over time. Approximately 40 percent of the damage to PCC pipes can be attributed to corrosion by biogenous sulphuric acid attack as a result of long flow periods and insufficient ventilation of wastewater.
Fig. 7.1
Precast Geopolymer Concrete Pipes
7.2 Structural Elements
Geopolymer concrete is used for the casting
of both columns and beams. The load capacity of geopolymer columns is
influenced by load eccentricity, concrete compressive strength values and
longitudinal reinforcement ratios. Decreased eccentricity loading and reinforcement
ratio increases favour an increase in overall column load capacity.
Fig.7.2
Geopolymer Columns After Demoulding.
Fig.7.3 Geopolymer beam(10.8m ) craned into position.
7.3 Pavements Geopolymer concrete is used for the construction of heat resistant pavement due to its thermal capacity. Pozzolan-based geopolymer cements do not readily decompose when exposed to high temperatures and appear to be more structurally stable under such conditions than PCC. Geopolymer cements utilize more and store less water from solution during particle reaction, and therefore, prevent aged dry shrinkage and strength degradation due to rapid water loss under extreme heat.
Fig.7.4
Placing Of Pavement Using Geopolymer Concrete.
7.4 Retaining Wall
A total of over fifty 40 MPa geopolymer
precast panels were used a retaining wall for a private residence. The panels
were up to 6 metres long by 2.4 metres wide and were designed to retain earth
pressure of 3 metres.
Fig.7.5
Precast Geopolymer Retaining Walls For A Private Residence.
7.5 Other Applications
According to Davidovits (1988), geopolymeric
materials have a wide range of applications in the field of industries such as
in the automobile and aerospace, nonferrous foundries and metallurgy, civil
engineering and plastic industries. The type of application of geopolymeric
materials is determined by the chemical structure in terms of the atomic ratio
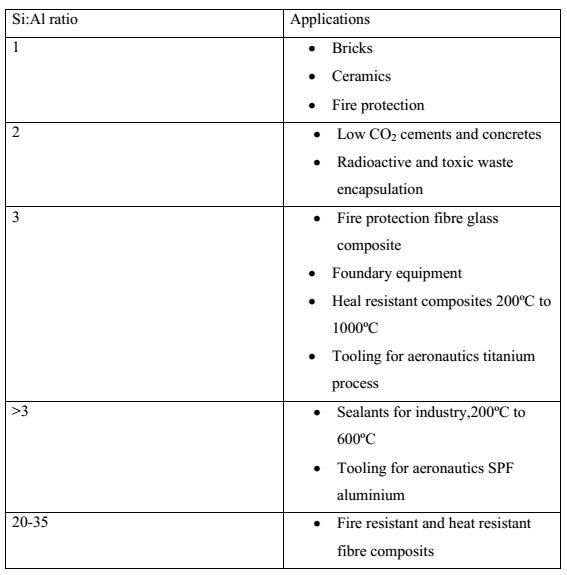
Si:Al in the polysialate. Davidovits (1999) classified the type of application
according to the Si:Al ratio as presented in Table 9.6 . A low ratio of Si:Al
of 1, 2, or 3 initiates a 3DNetwork that is very rigid, while Si:Al ratio
higher than 15 provides a polymeric character to the geopolymeric material.
Table 7.1 Application of geopolymeric materials based on Si:Al atomic ratio Si:Al ratio
8. CONCLUSION
Geopolymer concrete is well known for its
promising mechanical properties, acid resistance and fire resistance and
therefore is a potential alternative construction material with comparable
properties to OPC concrete. The constituents of Geopolymer Concrete shall be
capable of being mixed with a relatively low alkali activating solution and
must be curable in a reasonable time under ambient conditions. Geopolymers emit
approximately 80% less CO2 than OPC during production, making it a more
environmental friendly building material. Like OPC concrete, geopolymer
concrete has a brittle failure. Alternatively, fibres can be added to improve
the ductility of concrete. The properties of geopolymer include high early
strength, low shrinkage, freeze-thaw resistance, sulphate resistance and
corrosion resistance. These high-alkali binders do not generate any
alkali-aggregate reaction. The geopolymer binder is a low CO2 cementious material. It does not depend on the Calcination of
limestone that generates CO2. This technology can save up to 80% of CO2 emissions caused by the cement and aggregate industries. Due to
the high early strength, Geopolymer Concrete shall be effectively used in the
precast industries, so that huge production is possible in short duration of
time.
REFERENCES
1.Ammar Motorwala, Vineet Shah, Ravishankar kammula, Praveena Nannapaneni, Prof. D.B. Ranjiwala (2013), Alkali Activated Fly-Ash
Based Geopolymer Concrete, International Journal of Emerging Technology and
Advanced Engineering, Volume 3, Issue1,159-166.
2. Kolli Ramujee, M. Potharaju (2014), Development of
Low Calcium Flyash Based Geopolymer Concrete, IACSIT International Journal of Engineering
and Technology, Volume 6, Issue 1.
3. M.I Abdul Aleem, P.D.Arumairaj(2012), Geopolymer
Concrete –A Review, International Journal of Engineering Sciences &
Emerging Technologies, Volume 1, Issue 2,118-122.
4. P. Vignesh, A.R.
Krishnaraja, N. Nandhini (2014), Study on Mechanical Properties of Geopolymer concrete using M-sand and Glass Fibers,
International Journal of Innovative Research in Science, Engineering and
Technology, Volume 3, Issue 2, 110-116.
5. Raijiwala D.B, Patil H.S (2011), Geopolymer Concrete:
A Concrete Of Next Decade, Journal of Engineering Research and Studies, Volume 2,
Issue 1,19-25.
6. S.E Wallah , B.V.Rangan (2006), Low Calcium Fly Ash
Based Geopolymer Concrete: Long Term Properties, Curtin University of Technology, Perth ,
Australia.
7. Tawatchai Tho-in, Vanchai Sata, Prinya chindaprasirt, Chai
Jaturapitakkul (2012), Pervious High Calcium Flyash Geopolymer Concrete,
ELSEVIER Construction And Building Material, Volume 25, Issue 1,366-371.























No comments:
Post a Comment